World's Most Useful Molecule? - Kesler Science Weekly Phenomenon
Many years ago, chemistry was basically a spectator sport. Scientists observed substances found in nature to see how they reacted and describe their properties. Eventually, they learned to separate naturally occurring mixtures into pure compounds. They were limited in what they could do, though. Fuels, building materials, medicines, and even clothing dyes all had to come from natural sources. The idea of designing a compound from scratch was out of the question.
Then came benzene (C6H6) - the molecule that changed chemistry forever. It was important for two reasons. First, its ring structure was a groundbreaking discovery, as scientists thought molecules only occurred as long chains before that time. More importantly, chemists found they could adjust components attached to the carbon ring!
Benzene became the starting point for a whole new kind of chemistry. Scientists started to develop all sorts of useful chemicals that had never existed before - chemicals used in everything from clothing fibers and dyes to plastics and fuel additives. The field of synthetic chemistry was born.
These days, we know benzene is a very toxic material that should be avoided, but it will never lose its place in history. It's intriguing to think how one molecule totally changed our world. Could this kind of "chemistry revolution" ever happen again? Many scientists say it's happening now!
The science world is buzzing about metal organic frameworks, or MOFs. The first MOF that caught everyone's attention was built way back in 1989 to mimic the crystal structure of diamonds. Diamonds are so strong because they are made of a repeating structure of carbon atoms.
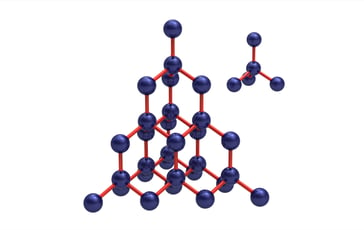
Instead of using carbon, the chemist built a similar pattern using metal copper atoms and organic nitrile molecules - that's why they're called metal-organic frameworks.
The other difference between diamond and the MOF was that the MOF's copper and nitrile pattern left a lot of empty spaces inside the structure. Unlike diamond, which is too dense to absorb anything, the spaces in the MOF can act like the holes in a sponge. Just like a sponge can soak up and hold a liquid in its pores, the MOF could collect and store liquids and gases.
Scientists have been busy since that first MOF was created! Now they can use other metals and other organic molecules to create strings and sheets of MOFs that capture all sorts of materials. One type of MOF is used in deserts to capture water molecules in the cool night air. When the sun comes up and warms the strings of MOFs, they expand and drop the water into collection tubs!
Part of the reason MOFs are so useful is that they can be custom-built to hold different sizes of molecules in their gaps. That means, for example, that a water filter made of MOFs can capture benzene but let water flow through.
MOFs are also useful because they hold a lot of material compared to their size. One MOF creator said that just one gram of a certain MOF would have a total surface area of more than two football fields. 🏈 That's a lot of storage!
Chemists are seeing all sorts of ways MOFs could be used to help solve problems on a global scale: latching on to greenhouse gases in the atmosphere, soaking up oil from tanker spills, and saving valuable rare earth metals from industrial waste.
MOFs are also really great at passing electrical signals and could improve wearable technology like smart watches.
The list of possibilities is long, and scientists are excited. In fact, two pioneering MOF inventors just won the Nobel Prize in Chemistry!
The graph below shows the number of new MOFs registered each year in the Cambridge Structural Database.
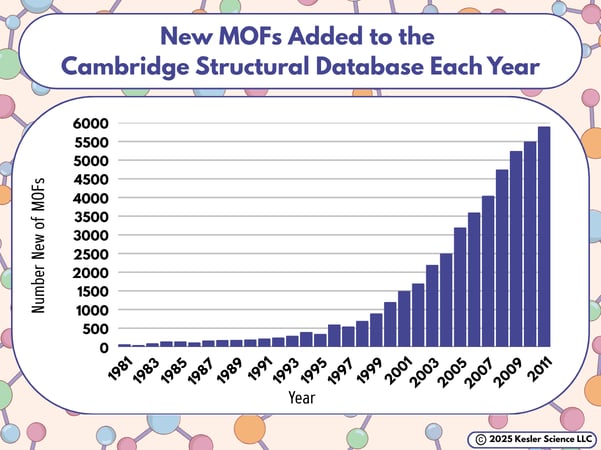
If I brought this graph into the classroom, here are some questions I'd have to go with it:
💡Identify the independent variable and dependent variable in this graph. (Hint: the independent variable is the one that researchers change in a consistent way, and the dependent variable is the one they watch for results.)
💡What trends do you notice in the number of new MOFs featured in this graph? Accept reasonable responses.
💡Based on the data in the graph, can you tell what the number of total MOFs were in the Cambridge Structural Database as of 2011?



.jpg?width=352&name=Jeju_Island_20141128_01_(15897128172).jpg)