Unexpected Earth - Moon Connection - Kesler Science Weekly Phenomenon
The relationship between the Moon and Earth is fascinating. Most schools teach how the Moon creates tides on our planet, but there is so much more going on! For example, Earth might actually be causing a type of rust to form on the Moon.
Rust is everywhere on our planet, ruining cars, boats, and metal almost anywhere you look. But what is it?
When iron and oxygen are around water, they react to form the notorious dusty red stuff we call rust. The water in the reaction can be liquid or water vapor in the atmosphere. Because our planet is so full of oxygen and water, rust forms easily here.
Rust on the Moon is surprising for two reasons. First, oxygen and water are in short supply on the Moon! There are no bodies of liquid water, and the thin atmosphere has almost no oxygen. Second, the Moon has no magnetosphere to protect it from the Sun's solar wind. The particles that flow from the Sun in the solar wind are full of hydrogen, which works against the formation of rust.
So how did the rust get on the Moon? India's lunar probe Chandrayaan collected data that experts found interesting. They think oxygen is traveling all the way from Earth's upper atmosphere to trigger the chemical reactions on the Moon!
How does oxygen from Earth get to the Moon, about 384,000 km away? Around the full moon phase of the lunar cycle, the Moon spends a few days in Earth’s magnetotail. The magnetotail is where the solar wind pushes Earth's magnetosphere out and away from the planet. The magnetotail is huge, stretching 2 million kilometers into space!
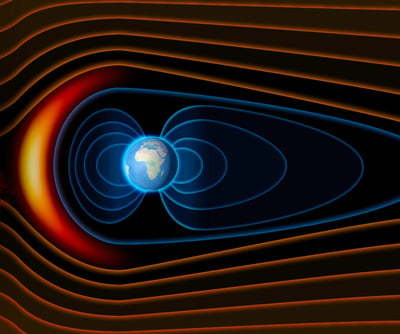
Inside the magnetotail, the Moon is shielded from the hydrogen particles coming from the Sun. Instead, a stream of particles from Earth, including oxygen atoms, flows to the Moon. Scientists think that when the energetic particles hit the Moon's surface, they cause tiny bits of ice hidden in lunar dust to melt. This might provide enough oxygen and water for the iron-rich minerals on the Moon's surface to start forming rust!
While this is just a hypothesis for now, there is some evidence that supports the idea. The far side of the Moon, which faces away from the Earth, never faces into the magnetotail. If the hypothesis is correct, there would be less rust forming on that side because there would be less oxygen and water available. When they looked at the data, scientists did find that, as predicted, the surface of the far side of the Moon has less rust than the surface that faces our planet.
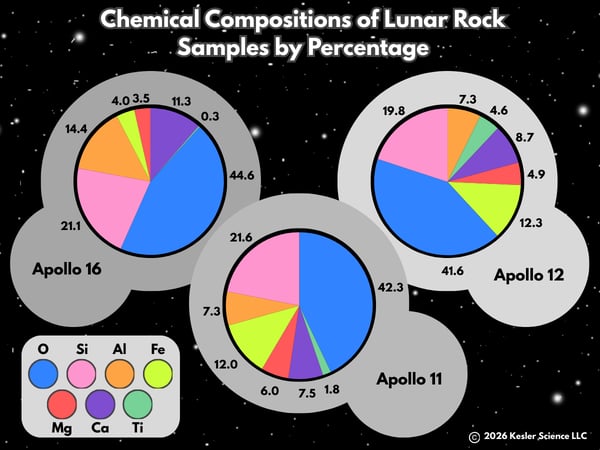
Ready to dig deeper into Moon rocks? The three pie charts below show the percentage of elements found in lunar rock samples collected from three different Apollo Moon missions. Take a look:
If I brought these graphs into my classroom, here are some questions I'd have to go with them:
💡Elements are known by their name and symbol, like the element oxygen is known by the "O" symbol. The other elements in the graphs are silicon (Si), aluminum (Al), iron (Fe), magnesium (Mg), calcium (Ca), and titanium (Ti). Which of the missions brought back samples with the highest percentage of magnesium? How can you tell?
💡The element oxygen floats free in the atmosphere, but it is also found in compounds like water, carbon dioxide, and iron oxide (another name for rust). Earth's crust is made up of 46% oxygen by weight. Which mission brought back samples closest to Earth's in oxygen?
💡Rust is made when iron and oxygen react in the presence of water. Which of the samples would be most likely to make rust with oxygen from Earth? Why do you say so?